|
What is an ACRONYM? According to Merriam-Webster's Collegiate dictionary, an acronym is: “a word (as NATO, radar, or laser) formed from the initial letter or letters of each of the successive parts or major parts of a compound term; also : an abbreviation (as FBI) formed from initial letters” | ||
| Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | ||
| ΔP | Delta Pressure, Change in pressure between two or more areas. | |
| µ | Micron or Micrometer ((µm, often abbreviated as um, micron or µ) A unit of length; one-millionth of a meter or 10,000 angstroms.) | |
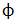 | Electrical Phase Symbol | |
| 356h | FDA Acronym (Drug applications) - New Drug Application | |
| 482 | FDA Acronym (Inspection forms) - Notice of Inspection form | |
| 483 | FDA Acronym (Inspection forms) - Inspectional Observations form | |
| 484 | FDA Acronym (Inspection forms) - Receipt of Samples form | |
| 510(k) | FDA Acronym (Device applications) - premarket notification | |
| A | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| AATB | American Association of Tissue Banks | |
| ACH | Air Change per Hour | |
| ACL | Computer Acronym - Access Control Level -or- Access Control List | |
| AE | Adverse Event | |
| AGAB | PDA Acronym - Audit Guidance Advisory Board | |
| ALCOA | FDA Acronym - Attributable, Legible, Contemporaneous, Original, Accurate | |
| ANDA | FDA Acronym (Drug applications) - Abbreviated New Drug Application | |
| ANPRM | Advance Notice of Proposed Rule Making (FDA) | |
| ANSI | American National Standards Institute | |
| API | Active Pharmaceutical Ingredient | |
| APA | Aseptic Processing Area | |
| APS | Aseptic Processing Simulation | |
| AQL | Acceptable Quality Level | |
| ASHRAE | American Society of Heating, Refrigerating and Air-Conditioning Engineers | |
| ASME | American Society of Mechanical Engineers | |
| ASTM | American Society for Testing and Materials | |
| AVL | Computer Acronym - Approved Vendor List | |
| B | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| BAS | Building Automation System | |
| BBP | Bloodborne Pathogens | |
| BiB | FDA Acronym - Bioinformatics Board | |
| BioAB | PDA Acronym - Biotechnology Advisory Board | |
| BLA | FDA Acronym (Drug applications) - biologics license application | |
| BMCS | Building Management and Control System | |
| BMS | Building Management System | |
| BOCA | Building Officals and Code Administrators International | |
| BOD | Basis of Design | |
| BOM | Computer Acronym - Bills of Materials | |
| BOP | Batch Operating Procedure | |
| BRBs | FDA Acronym - Business Review Boards | |
| BSC | Biological Saftey Cabinet | |
| C | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| CAD | Computer Acronym - Computer Aided Design | |
| CAM | Computer Acronym - Computer Aided Manufacturing | |
| CAPA | Corrective and Preventive Action | |
| CAx | Computer Acronym - Product Design | |
| CBER | Center for Biologics Evaluation and Research | |
| CCR | Change Control Request | |
| CDASH | FDA Acronym - Clinical Data Acquisition Standards Harmonization | |
| CDER | Center for Drug Evaluation and Research | |
| CDISC | FDA Acronym - Clinical Data Interchange Standards Consortium | |
| CDRH | Center for Devices and Radiological Health | |
| cfm | Cubic Feet per Minute | |
| CFR | Code of Federal Regulations | |
| 21CFR, | Code of Federal Regulations - Section 211.25 Signatures | |
| 21CFR, | Code of Federal Regulations - Section 211.46 Disaster Recovery | |
| CFSAN | FDA Acronym (FDA centers and offices) - Center for Food Safety and Applied Nutrition | |
| CFU | Colony Forming Unit | |
| cGAP | current Good Auditing Practice | |
| cGBPs | current Good Business Practices | |
| cGDP | current Good Documentation Practice | |
| cGISP | current Good Information Systems Practice | |
| cGMP | current Good Management Practice | |
| cGMP | current Good Manufacturing Practice | |
| cGSP | current Good Safety Practice | |
| CGTP | FDA Acronym - Current Good Tissue Practice | |
| CIP | Clean In Place - applies to cleaning equipment, vessels +/or piping that's too large to disassemble in order to clean. | |
| CMC | FDA Acronym (Drug applications) - chemistry, manufacturing, and controls | |
| CMO | Contract Manufacturing Organization | |
| COC | Chain of Custody | |
| COP | Clean Out of Place - applies to parts, equipment or vessels that are removed from their operating locations to be cleaned. | |
| COP | Communities of Practice. (Relating to GAMP) | |
| COTS | Commercial Off The Shelf | |
| CPGM | FDA Acronym - Compliance Program Guidance Manual | |
| CRM | Computer Acronym - Customer Relationship Management | |
| CRM:248 | State Specific - Code of Massachusetts Regulations - Plumbing Codes | |
| CRM:310 | State Specific - Code of Massachusetts Regulations - Environmental Protection requirements | |
| CRO | FDA Acronym (Contract organizations) - Contract Research Organization | |
| CSV | FDA Acronym - Computer System Validation | |
| CTU | Control Temperature Unit | |
| CV | CurriculaVitae | |
| CVM | FDA Acronym (FDA centers and offices) - Center for Veterinary Medicine | |
| D | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| D&E | FDA Acronym - Development and Execution | |
| DAARTS | FDA Acronym - Workflow Tracking and Information Management System | |
| DAS | Data Acquisition System | |
| DCS | Document Control System | |
| DDS | Detailed Design Specification | |
| DFM | Computer Acronym - Design For Manufacturing | |
| DHRs | Computer Acronym - Device History Records | |
| DMF | FDA Acronym (Drug applications) - drug master file | |
| DMM | Digital Multimeter | |
| DO | FDA Acronym (FDA centers and offices) - District Office | |
| DOE | Design of Experiment | |
| DOP | Dispersed Oil Particulate | |
| DOP | HEPA Filter Testing - Di-Octyl Phthalate | |
| DQ | Design Qualification | |
| E | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| E-Factor | PDA Acronym - Dutch Professor Roger Sheldon, Delft University of Technology, put the ´E´ in E-factor in the late 1980s. The E-Factor , or Environmental Factor, is the ratio of the mass of waste per unit of product. The factor is widely used for determining the efficiency and environmental impact of chemical processes. | |
| EA | FDA Acronym - Target Enterprise Architecture | |
| EC | European Community | |
| ECA | Environmentally Controlled Area | |
| ECMS | Computer Acronym - Electronic Content Management Systems | |
| ECR | Environmentally Controlled Room | |
| eCRF | FDA Acronym - Electronic Case Report Form | |
| eDHR | Computer Acronym - electronic Device History Record | |
| EDMS | Computer Acronym - Electronic Document Management Ssystems | |
| EDR | FDA Acronym - Electronic Document Room | |
| EER | Equipment Excursion Report | |
| EIR | FDA Acronym (Inspection forms) - Establishment Inspection Report | |
| EM | Environmental Monitoring | |
| EMR | Electronic Medical Record | |
| ERP | Computer Acronym - Enterprise Resource Planning | |
| ESG | FDA Acronym - Electronic Submissions Gateway | |
| EU | European Union | |
| F | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| FAT | Field Acceptance Test | |
| FAERS | FDA Acronym - FDA-wide Adverse Event Reporting System | |
| FDA | U.S.Food and Drug Administration | |
| FD&C | FDA Acronym - Act Food, Drug, and Cosmetic Act | |
| FDCA | FDA Acronym - Food, Drug, and Cosmetic Act | |
| FOI | FDA Acronym - freedom of information | |
| FOIA | FDA Acronym - Freedom of Information Act | |
| FQ | Facility Qualification | |
| FR | FDA Acronym - Federal Register | |
| FRS | Functional Requirements Specification | |
| FS/CS | Functional and Configuration Requirements Combined Specification | |
| ft3 | Cubic Feet | |
| G | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| GAC | Granular Activated Carbon | |
| GAMP | Good Automation Manufacturing Practices | |
| GAMP4 | Good Automation Manufacturing Practices Released 2001 | |
| GAMP5 | Good Automation Manufacturing Practices Released 2008 | |
| GCP | Good Clinical Practice | |
| GLP | Good Laboratory Practice | |
| GMP | Good Manufacturing Practice | |
| GPM | Gallons per Minute | |
| GTP | FDA Acronym - Good Tissue Practice | |
| GTAW | Welding Acronym - Gas Tungsten Arc Welding | |
| H | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| HEPA | High Efficiency Particle Air Filter | |
| HHS | FDA Acronym (FDA centers and offices) - U.S. Department of Health and Human Services | |
| HL7 | FDA Acronym - Health Level Seven | |
| HVAC | Heating, Ventilation and Air Conditioning | |
| Hz | Hertz (Number of cycles per second) | |
| I | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| IAC | Indoor Air Quality | |
| IB | FDA Acronym (FDA centers and offices) - Inspections Branch | |
| IC | Inorganic Carbon | |
| ICH | FDA Acronym - International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use | |
| ICH | International Conference on Harmonisation | |
| ICT21 | FDA Acronym - Information Computer Technology for the 21st century | |
| IDE | FDA Acronym (Device applications) - Investigational Device Exemption | |
| IEEE | Institute of Electrical and Electronic Engineers | |
| inAq | Inches of Water [1 inAq = .036126 psi, or 27.67936 inAq = 1 psi] | |
| IND | FDA Acronym (Drug applications) - Investigational New Drug application | |
| IOM | FDA Acronym - Inspections Operations Manual | |
| IPQ | PDA Acronym - International Pharaceutical Quality | |
| IQ | Installation Qualification | |
| IOQ | Installation/Operational Qualification | |
| ISO | International Standards Organization | |
| ISPE | International Society for Pharmaceutical Engineering | |
| J | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| K | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| L | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| LCC | Living Cell Construct | |
| LCD | Computer Acronym - Liquid Crystal Dispaly | |
| LOQ | Limit of Quantitation | |
| LPM | Liters Per Minute | |
| LQD | Limit of detection | |
| M | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| M3 | Cubic Meter | |
| MDR | Medical Device Reporting | |
| MES | Computer Acronym - Manufacturing Execution System | |
| MOC | Material of Construction | |
| MOU | PDA Acronym - Memorandum of Understanding | |
| MP | Master Protocol | |
| MPM | Computer Acronym - Manufacturing Planning Management | |
| MRP | Computer Acronym - Manufacturing Resource Planning | |
| MVP | Validation Acronym - Master Validation Plan | |
| N | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| N/A | Not Applicable | |
| NAI | FDA Acronym (Inspection forms) - No Action Indicated | |
| NB | National Board of Boiler and Pressure Vessel Inspectors | |
| NBBI | National Board of Boiler and Pressure Vessel Inspectors | |
| NCE | FDA Acronym (Drug applications) - new chemical entity | |
| NCLR | Non-Clinical Laboratory Study Report | |
| NCLVP | Non-Clinical Validation Protocol | |
| NCLVR | Non-Clinical Validation Report | |
| NCMR | Non-Conforming Material Report | |
| NDA | FDA Acronym (Drug applications) - New Drug Application | |
| NEC | National Electrical Code | |
| NEMA | National Electrical Manufacturers Association | |
| NFPA | National Fire Protection Code | |
| NIH | FDA Acronym (FDA centers and offices) - National Institutes of Health | |
| NIST | National Institutes of Standards and Technology | |
| NME | FDA Acronym (Drug applications) - new molecular entity | |
| NPD | Computer Acronym - New Product Development | |
| NRC | National Research Council | |
| O | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| OAI | FDA Acronym (Inspection forms) - Official Action Indicated | |
| OCC | FDA Acronym (FDA centers and offices) - Office of Chief Counsel | |
| OCR | Computer Acronym - Optical Character Recognition | |
| OECD | Organization for Economic Co-operation & Development | |
| OGC | FDA Acronym (FDA centers and offices) - Office of General Counsel | |
| OI | FDA Acronym (FDA centers and offices) - Office of Investigations | |
| OIG | FDA Acronym (FDA centers and offices) - Office of Inspector General | |
| OIT | Operator Interface Terminal | |
| OOS | FDA Acronym - Out Of Specification | |
| OOT | Out of Trend | |
| OQ | FDA Acronym - operational qualification | |
| OQ | Operational Qualification | |
| OPIM | Other Potentially Infectious Material | |
| OPQ | Operational/Performance Qualification | |
| ORA | Office of Regulatory Affairs | |
| OSHA | Occupational Health and Safety Administration | |
| P | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| PAC | Programmable Automation Controller | |
| PAPR | Powered Air Purifying Respirator | |
| PCR | Product Complaint Report | |
| PIC/S | Pharmaceutical Inspection Co-operation Scheme | |
| PD | Planned Deviation/Planned Departure | |
| PDM | Computer Acronym - Product Data Management | |
| PE | Process or Performance Evaluation | |
| PFA | Perfluoroalkoxyalkane (ASTM Spec. D3307) | |
| PLM | Computer Acronym - Product Lifecycle Management | |
| PM | Preventative Maintenance | |
| PMA | FDA Acronym (Device applications) - Premarket Approval | |
| PMM | Computer Acronym - Product and Portfolio Management | |
| PP | Polypropylene (ASTM Spec. D4101) | |
| PPE | Personal Protective Equipment | |
| ppm | Parts per Million | |
| PQ | FDA Acronym - performance qualification | |
| PQ | Performance Qualification | |
| psig | Pounds per Square Inch - gauge | |
| PTFE | Polytetrafluoroethylene (ASTM Spec. D4894 & D4895) | |
| PV | FDA Acronym - process validation | |
| PV | Process Variable | |
| PVDF | Polyvinylidene Fluoride (ASTM Spec. D3222) | |
| Q | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| QA | FDA Acronym - quality assurance | |
| QC | FDA Acronym - quality control | |
| QMS | Quality Management System | |
| QS | Latin - Quantum satis - meaning 'the amount which is needed' | |
| QS | FDA Acronym - quality system | |
| QSR | FDA Acronym - Quality System Regulation | |
| Qty | Quantity | |
| QU | FDA Acronym - quality unit | |
| R | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| rDNA | recombinant DNA | |
| RH | Relative Humidity | |
| RGA | Return Goods Authorization | |
| RIM | FDA Acronym - Reference Information Model | |
| RM | FDA Acronym (Drug applications) - raw material | |
| RMA | Return Material Authorization | |
| RPS | FDA Acronym - Regulated Product Submission | |
| RO | Reverse Osmosis | |
| RQ | Re-Qualification | |
| RS | Reference Standard | |
| RTU | Ready To Use | |
| RV | Re-Validation | |
| Rw | Reagent Water | |
| S | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| SAB | PDA Acronym - Science Advisory Board | |
| SAE | Serious Adverse Event | |
| SAT | Site Acceptance Test | |
| SCADA | Supervisory Control and Data Acquisition | |
| SCC | Standards Council of Canada | |
| SCFM | Standard Cubic Feet per Minute | |
| SDMP | Systems Development Master Plan | |
| SHFDA | PDA Acronym - Shanghai Municipal FDA | |
| SIFDS | PDA Acronym - Shanghai Institute for Food and Drug Safety | |
| SIP | Sterilize In Place - applies to sterilizing equipment, vessels +/or piping that's too large to fit into an autoclave. | |
| SISPQ | Safety, Identity, Strength, Purity and Quality | |
| SLC | System Life Cycle | |
| SLPM | Standard Liters Per Minute | |
| SMACNA | Building Acronym - Sheet Metal and Air Conditioning Contractors' National Association | |
| SOA | FDA Acronym - Service Oriented Architecture | |
| SOP | Standard Operating Procedure | |
| SSS | Sucrose Stock Standard | |
| T | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| TC | Total Carbon | |
| TOC | Total Organic Carbon - a basic, broadband measure of cleanliness. Also one of the critical measurements of water quality. | |
| TOP | Turn Over Package. | |
| TPD | Total Percent Defective | |
| TRI | PDA Acronym - Training and Reasearch Institute | |
| TSA | Microbiological Media - Tryptic Soy Agar | |
| TSB | Microbiological Media - Tryptic Soy Broth | |
| U | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| UAT | Computer Acronym - User Acceptance Testing | |
| UD | Un-planned Deviation/Un-planned Departure | |
| UK | United Kingdom | |
| uL | microliter/microliters | |
| UL | FDA Acronym (Inspection forms) - untitled letter | |
| UL | Underwriters Laboratories | |
| UNII | FDA Acronym - Unique Ingredient Identifiers | |
| UPS | Computer Acronym - Uninterruptible Power Supply | |
| UQL | Unacceptable Quality Level | |
| URS | User Requirements Specification | |
| USA | United States of America | |
| USP | Unitied States Pharmacopeia | |
| USPNF | FDA Acronym - United States Pharmacopeia National Formulary | |
| V | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| VAI | FDA Acronym (Inspection forms) - Voluntary Action Indicated | |
| VFD | Variable Frequency Drive | |
| VFR | Validation Final Report | |
| VMP | FDA Acronym - validation master plan | |
| VPDR | Validation Acronym - Validation Protocol Discrepancy Report | |
| VP | Validation Acronym - Validation Protocol | |
| VPP | Validation Acronym - Validation Project Plan | |
| VRA | Validation Acronym - Validation Risk Assessment | |
| W | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| WFI | Water for Injection - high purity water used to formulate and clean in biotech and pharmaceutical processes. | |
| WIP | Computer Acronym - Work in Process | |
| WL | FDA Acronym (Inspection forms) - warning letter | |
| WOG | Water-Oil-Gas A rating designation generally used for small valves chiefly in low ratings. Indicates maximum working pressure at ambient + 32oF to +100oF. | |
| X | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| Y | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |
| Z | Top / Bottom / A B C D E F G H I J K L M N O P Q R S T U V W X Y Z | |